|
T R U T O N
|
|
The
Rational
Unified
Theory
Of
Nature
|
by
Kalman
Klim Brattman
|
Give
me the simplest form of
matter
and motion,
and I will build, out of them, the world
of Nature.
|
|
|
"Give me matter,
and I will construct a world out of it."
Immanuel
Kant, Kant's Cosmology
("Universal
Natural History and Theory Of
Heavens")
|
|
12.
Atoms, Their Nucleus And Electron
Configuration
|
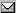
|
|
 So
far, we have introduced the simplest and most
abundant atomic nucleus --that of the Hydrogen
consisting of a single proton. However, the
situation complicates itself immensely when we
consider nuclei containing more than one proton.
And that is because of their periodic
outflux
bursts,
protons by repelling themselves will not be able
to form a stable unit.
So
far, we have introduced the simplest and most
abundant atomic nucleus --that of the Hydrogen
consisting of a single proton. However, the
situation complicates itself immensely when we
consider nuclei containing more than one proton.
And that is because of their periodic
outflux
bursts,
protons by repelling themselves will not be able
to form a stable unit.
In
the current speculative "new" Physics, to
overcome the impasse created by the repelling
protons, it was postulated, out-of-the-blue, the
existence of a so-called "strong force"
capable of keeping them together. That
speculative force, of unknown origin, acting
only at a short distance, was purported to be
mediated by another speculative particle
[sic!] --the meson coined in
1934-36 by Hideki Yukawa in conjunction
with Werner
Heisenberg.
In
TRUTON, it goes without saying, that such a
speculative approach is both pathetic and
repugnant.

Example
of the simplest
stability
configuration.
|
In introducing the
protons
(PRs),
we have recognized that only equiphased
protons
repel themselves. The outphased
protons,
on the other hand, attract themselves. The
problem however is that when two
outphased
protons are
in contact, they both become
equiphased.
As such, to preserve their respective
outphased
states, protons
must be separated by neutral buffer bridges that
neutrons
(NTs)
can provide.

Doubled
Interlinked
Bridge
(DIB).
|

|
 A neutron
(NT)
in contact and positioned between two (2)
protons is said to form an interlink with
them and call that singular
interlink
the neutron buffer (neb)
link (neblink). If two (2) protons are
interlinked
with two (2) neblinks,
then they are said to be interconnected with a
doubled interlinked bridge (DIB). A
DIB
(that contains two neblinks)
between two protons, offers for them obviously a
much stronger and resilient
interlink
than the one of a single
neblink.
A neutron
(NT)
in contact and positioned between two (2)
protons is said to form an interlink with
them and call that singular
interlink
the neutron buffer (neb)
link (neblink). If two (2) protons are
interlinked
with two (2) neblinks,
then they are said to be interconnected with a
doubled interlinked bridge (DIB). A
DIB
(that contains two neblinks)
between two protons, offers for them obviously a
much stronger and resilient
interlink
than the one of a single
neblink.

|
.
|

|
In an atomic
nucleus, called nucletron
(or
in short, a NUC),
its protons are said to be interconnected
through their neblinks.
(Using a mathematical jargon, we say that the
protons of a NUC
are all interconnected
modulo
neblinks.)
In general, for a NUC
to be stable, its protons and neutrons must
arrange themselves in such a way that each
proton-pair be interconnected
with a DIB.
A rare exception however exists for Helium
whose two protons, can be interlinked only
with one neblink,
to form the rare stable
NUC
of the Helium-3.
The most common stable variant of
Helium
being however, as expected from TRUTON, the
Helium-4
whose two protons are
interconnected
with a DIB
rather than with a neblink.
|
Recap
On the Structure of a Stable Nucletron
(NUC)
|
|
1.
All stable NUCs
are made of outphased
protons
that attract each other.
|
|
2.
Equiphased
protons
by repelling each other cannot be
part of any stable NUC.
|
|
3.
The outphased
protons
that are in contact become
equiphased
and, as such, they cannot be part of a
NUC.
Because of that, the outphased protons
of a NUC
must have between them
neutron-buffers
(nebs).
|
|
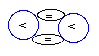
Alpha-
particle
that is identical to the
Helium-4 NUC.
|
|

|
|
|
Ernest
Rutherford
|
4.
The simplest and most abundant stable
formation is the one made of two
protons that are interlinked through a
doubled
interlinked bridge
(DIB).
Those populous particles that are
interlinked
with a
DIB,
called alpha particles
(discovered and named by
Ernest
Rutherford),
are in fact the NUCs
of
the mentioned Helium-4.
|
|
5.
The
evenness or oddness of the number of
protons and neutrons of a long-lived
nucletron
(NUC)
must plays an important factor in its
stability, with the odd
NUCs
to be far less stable. And that is
because of the DIBs
that
are made of a pair of neblinks.
|

|
|
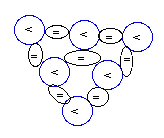
NUC-Diagrams'
Legend:
"<"--Proton;
"="--Neutron
|
|
The
NUCs of the first six Chemical
Elements
|
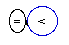
|
Deuterium
[rare stable isotope]
|
Hydrogen
(1H) by having only one proton in
its nucleus, called also protium, has
another stable isotope, called deuterium
(2H), that contains one proton
and one neutron. The naturally occurring isotope
containing two neutrons, called tritium,
is an unstable radioactive element, being thus a
degenerated element of Nature.
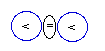
|
|

|
Helium-3
(3He)
[stable and less abundant ]
|
|
Helium-4
(4He)
[stable and most abundant
]
|
Helium
(2He) with two protons in its
nucleus, has two stable isotopes. Its most
abundant isotope is the one that contains a
DIB
in its nucleus, generating thus the formation of
2 protons (2P) and 2 neuteons (2N), aka
(2P+2N), denoted as 4He. Its rarest
stable isotope is the one containing not the
DIB,
but the singular neblink
(2P+1N).
That rare isotope, denoted as 3He, is
difficult to be formed because of its
NUC
configuration of having two outphased
protons
(2P) interlinked
with
only one neblink.
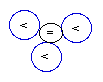
|
|
|
|
|
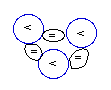
Lithium-4
(4Li)
[unstable]
|
|
Lithium-6
(6Li)
[stable and less abundant
]
|
|
Lithium-7
(7Li)
[stable and more abundant ]
|
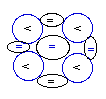
Lithium
(3Li) has 3 protons (P). The
NUC
with 4 neutrons that is (3P+4N) generates by far
its most stable isotope (denoted as
7Li), because with that formation
each proton can be anchored with any other
proton through three (3) neblinks.
The other stable, but less abundant isotope
(denoted as 6Li), has its
NUC
composed of 3N+3P can generate only two (2)
neblinks
for
each of its 3 protons (P).
|
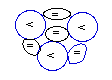
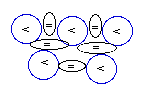
Beryllium-9:
4P+5N
[sole stable isotope]
|
Beryllium
(4Be) has 4 protons. Its sole
possible isotope, Beryllium-9 (9Be),
is the one whose NUC
has 5 neutrons that are able to establish three
(3) neblinks
for each proton (<) as represented in the
diagram above.
|
|
|
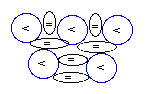
Boron-10:
(10B)
[stable and less abundant ]
|
|
Boron-11:
(11B)
[stable and more abundant ]
|
Boron
(5B) with 5 protons has two
stable isotopes:
|
the
one, with 5 neutrons (5P+5N) --the B-10
(10B) and,
the more abundant one (because of its
greater stability), with 6 neutrons
(5P+6N) --the B-11 (11B), as
represented in the diagram
above.
|
|
|
|
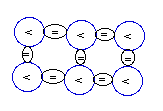
Carbon-12
(12C):
6P+6N
[stable and most abundant ]
|
|
Carbon-13
(13C):
6P+7N
[stable and far less abundant ]
|
Carbon
(6C) with 6 protons has two
stable isotopes: the one with 6 protons and the
other more rare with 7 protons as represented in
the diagram above.
|

Niels
Bohr
|
On
the Electron Configuration (ELcon)
within the
Atom

The
Rutherford-Bohr Transcendent (RUBOT)
Model
|

Ernest Rutherford
|
 While
the structure of a stable atomic nucleus
(NUC)
is relatively simple, being a proton-neutron
interplay following established geometrical
patters to ensure that its protons remain
outphased,
the electron configuration (ELcon) within
the atom, on the other hand, is considerable
more complex. While
the structure of a stable atomic nucleus
(NUC)
is relatively simple, being a proton-neutron
interplay following established geometrical
patters to ensure that its protons remain
outphased,
the electron configuration (ELcon) within
the atom, on the other hand, is considerable
more complex.
Each
outphased proton of a spinning NUC
(whose spin was created, at its very formation,
from various formative collisions) will generate
an uneven undulated globular inflow field-wave
called the wavelon ball (wab) around it.
That wab
will have, in it, a circular "valley" --called
valon-- that is shaped by the proton's
influx-outflux
cycle.
Through
the field superimposition,
NUCs
with more than one outphased proton, would
create around them circular
stratified "valleys" (aka
valons)
whose
distance from the NUC
will increase --by superimposition-- with the
increase of number of its protons. The "loose"
or "free" electrons from a NUC's surrondings
will be sucked-in through Downlev
(by
the inflow field generated by the NUC's protons)
into the existing valons.
Those electrons, in addition, will acquire a a
back-and-forth oscillatory spin (ospin)
due to the different angles and the strength of
attraction coming from the NUC.
 When
the 1st
stratified valon
become completely occupied, the remaining "free"
electrons will be pushed by the electrons of the
1st valon
to the subsequent 2nd
stratified valon,
and so on. That electron-push is done through
the electron's inherent XB-cloud.
We can talk, as such, about the electron
packing (ELpack) of a
valon
and of a maximum number of electrons that can be
packed into a valon
called maxpack.
When
the 1st
stratified valon
become completely occupied, the remaining "free"
electrons will be pushed by the electrons of the
1st valon
to the subsequent 2nd
stratified valon,
and so on. That electron-push is done through
the electron's inherent XB-cloud.
We can talk, as such, about the electron
packing (ELpack) of a
valon
and of a maximum number of electrons that can be
packed into a valon
called maxpack.

The ELpack Saturation Theorem
(TELSAT)
|
Maxpacks do not depend on the size of
valons.
|
|
 Proof:
Proof:
We
begin with the recognition
that the electron's XB-cloud
varies
in size with the
ergolevel
(erL)
of its surrounding environment (being
smaller, i.e., being shrunken more),
the higher the density of its
environmental xenofluid
(eXF).
To
this, we add another recognition,
namely that the field-density of a
valon
decreases proportionally with
the distance from the NUC.
The
proportionality between the
valon's
field rarefaction and the electron's
XB-cloud
variation
is the key in here.
QED.
|
A
valon
that is packed to its fullest is said to be
saturated (SAT). The unsaturated
(UNSAT) valons, on the other hand, are those
that have room in accepting additional
electrons. The exact numbers of electrons
required for a particular valon
to become saturated
(i.e., the maxpack)
is a number that only can be obtained from
experimental data.
 In
the next page, with the use of experimental data
and additional inferences of the
electron
configuration (ELcon)
of atoms,
a magic maxpack
number eight (8) will pop-out as
being a preferred tendency for many atoms to
acquire for their outer valon
(called ovalon). A locked-in, most stable
saturated ovalon (satov) of
eight (8) electrons is being
created. In
the next page, with the use of experimental data
and additional inferences of the
electron
configuration (ELcon)
of atoms,
a magic maxpack
number eight (8) will pop-out as
being a preferred tendency for many atoms to
acquire for their outer valon
(called ovalon). A locked-in, most stable
saturated ovalon (satov) of
eight (8) electrons is being
created.
Now
since the wab
has its "roots" into the spinning
NUC
from where it emerged, it follows that the
wab
(with its valons)
will spin together. The residing embedded
electrons
(ELs)
into the valons,
will thus be dragged to rotate around the
NUC
generating, as such, circular tracks called
the electrons' rings (elrings or, in
short, elR).
 A radically new picture begins now to emerge:
the ospinning
electrons
(ELs)
residing in the rotating valons
will begin, from their inception, to rotate
around the NUC, creating the elrings,
as
part of their spinning wab.
A radically new picture begins now to emerge:
the ospinning
electrons
(ELs)
residing in the rotating valons
will begin, from their inception, to rotate
around the NUC, creating the elrings,
as
part of their spinning wab.

On the Chemical Elements Variants
(CEVs) in Nature
|
  A
pair of protons of a stable NUC,
of
course, can be interconnected
not only with a NEB
but
also, in addition or separately, with a
pab,
generating, as such, the neutron-variants
(nevas) of a stable
NUC,
called isotopes. Thus, to a particular
chemical element (chemel), we can
associate its corresponding
istopes.
For instance, the mentioned Helium-3
and Helium-4
are said to be the isotopes
of the Helium. A
pair of protons of a stable NUC,
of
course, can be interconnected
not only with a NEB
but
also, in addition or separately, with a
pab,
generating, as such, the neutron-variants
(nevas) of a stable
NUC,
called isotopes. Thus, to a particular
chemical element (chemel), we can
associate its corresponding
istopes.
For instance, the mentioned Helium-3
and Helium-4
are said to be the isotopes
of the Helium.
 Stable
NUCs
cannot increase in size indefinitely. And that
is because, as already noted,
there is a finite maximum number (maxin)
of permanent outphased
protons
that can exist for an
atomic
nucleus (NUC) to
remain stable. And that
maxin
was found experimentally to be 82 corresponding
to the chemical element Lead (in Latin
plumbum and denoted with the symbol
82Pb). Stable
NUCs
cannot increase in size indefinitely. And that
is because, as already noted,
there is a finite maximum number (maxin)
of permanent outphased
protons
that can exist for an
atomic
nucleus (NUC) to
remain stable. And that
maxin
was found experimentally to be 82 corresponding
to the chemical element Lead (in Latin
plumbum and denoted with the symbol
82Pb).
 Natural
radioactivity (narad)
begins with atoms whose NUCs
contains at least two distant equiphased
protons.
The
more equiphased
protons
an
atom has, the more radioactive or instable it
becomes. As such, narad
begins with the next chemical element following
the Lead
(82Pb)
that is the Bismuth (83Bi)
which, by being the 1st radioactive element, is
extremely weak radioactive.
Natural
radioactivity (narad)
begins with atoms whose NUCs
contains at least two distant equiphased
protons.
The
more equiphased
protons
an
atom has, the more radioactive or instable it
becomes. As such, narad
begins with the next chemical element following
the Lead
(82Pb)
that is the Bismuth (83Bi)
which, by being the 1st radioactive element, is
extremely weak radioactive.

F.
Soddy
|

M.
Todd
|
.
|
An
atom, i.e., a
chemical
element (chemel),
is defined by the number of protons its
NUC
has. However, the number of
neutron-buffers
that
a chemical element has can vary within
certain limits dictated by its
stability requirement. Those
neutron-variants
(nevas)
of a chemical element are called, as
stated above, its isotopes.
The word 'isotope' was introduced in
1913 by the English radiochemist
Frederick Soddy at the
suggestion of the Scottish MD and
writter Margaret
Todd.
|
|
 •
The natural stable isotopes
are indeed the embryonal
players of Nature (epons).
•
The natural stable isotopes
are indeed the embryonal
players of Nature (epons).
•
 The
short-lived, unstable isotopes,
on the other hand, are the "rejects"
of Nature (rons). However, because
of their abundance,
rons
play indeed a significant role in
shaping up Nature creating the
so-called cosmic rays
(corays). The
short-lived, unstable isotopes,
on the other hand, are the "rejects"
of Nature (rons). However, because
of their abundance,
rons
play indeed a significant role in
shaping up Nature creating the
so-called cosmic rays
(corays).
|
 As
with respect to the electron-variants
(elvas) within an
atom,
a proton can attract more than one electron and
as such, a NUC
can hold more electrons than the number of its
protons, resulting thus in the creation of
negatively charged chemical elements. As
with respect to the electron-variants
(elvas) within an
atom,
a proton can attract more than one electron and
as such, a NUC
can hold more electrons than the number of its
protons, resulting thus in the creation of
negatively charged chemical elements.
 In
a reverse,
a chemical element, once formed, could be
subjected to a "bombardment" of radiation from
its environment creating a situation where some
its electrons would be knocked out, creating now
a positively charged chemical element.
Thus, through an environmental bombardment
(enbo), depending of its intensity, a
chemical element may acquire or loose one or
more electrons transforming itself from a
neutral particle into a charged one. Those
electron-variants
(elvas)
of a chemical
element (chemel)
are called its ions.
In
a reverse,
a chemical element, once formed, could be
subjected to a "bombardment" of radiation from
its environment creating a situation where some
its electrons would be knocked out, creating now
a positively charged chemical element.
Thus, through an environmental bombardment
(enbo), depending of its intensity, a
chemical element may acquire or loose one or
more electrons transforming itself from a
neutral particle into a charged one. Those
electron-variants
(elvas)
of a chemical
element (chemel)
are called its ions.
|

M.
Faraday
|
 When through that
enbo,
a chemel
acquires
one or more free electrons, it will
transform itself into a negatively
charged chemel
-- that is, a negatively charged
ion
called an anion. And, to the
contrary,
When through that
enbo,
a chemel
acquires
one or more free electrons, it will
transform itself into a negatively
charged chemel
-- that is, a negatively charged
ion
called an anion. And, to the
contrary,
 When through that
enbo,
a chemel
looses
--by being knocked out-- one ore more
of its electrons, it will transform
itself into a positively charged
chemel
--that
is, a positively charged
ion
called a cation.
When through that
enbo,
a chemel
looses
--by being knocked out-- one ore more
of its electrons, it will transform
itself into a positively charged
chemel
--that
is, a positively charged
ion
called a cation.
All those concepts of
ions,
with the anion
and cation,
were introduced in 1834 by
Michael
Faraday.
|
Thus,
to a chemical
element (chemel),
we can associate its double sided variants: the
isotopes
and its charged ions
(i.e.,
its anions
and cations).
.Unlike
the proton
(PR),
the neutron
(NT)
is a composite unit formed out of a high-speed
collision between a proton and an electron
resulting in the formation of a unit made of a
naked
proton (nakep)
and a naked
electron (nakel)
--particle stripped of their charges. Thus,
for the neutron
--as a composite particle, unlike as for the
proton
that is not [sic!],
we need to entertain the problem of its
stability.
On
the Dual
Stability/Instability
Characteristics of the
Neutron
While
In/Out
of Atomic Nuclei
(NUCs)
|
The neutron
(NT)
is devoid of a charge, being thus neutral,
because its constituents --the
naked
proton (nakep)
and the
naked electron (nakel)
have been stripped of their respective
charges.
The
key player in determining the Neutron's
stability/instability is Downlev
that operate only above the ergobase
(erB)
line. Now, since the
ergolib
(erLib)
of the NUC
is below the ergobase
(erB),
it follows as already noted,
that Downlev
cannot reach the NUC.
As such, inside the NUC, the Neutron
is safe and stable.
For
a Neutron outside of the NUC, we have a
different situation, as there the
environmental xenofluid (eXF), by being
above the ergobase (erB)
line, will force --through
Downlev
-- the Neutron to break-up.
In
short, the limitation of Downlev
to
reach the atom assures the
neutron's
stability in the NUC.
We repeat this important result as follows:
- Inside
the atom, the
neutron
together with the outphased
protons
form the NUC-unit
that is below the BALE
ergoline and, as such, it is not
subject to the influence of
Downlev.
And that is because the
outphased
protons
form, collectively, a permanent
ergoHole
(erH)
entity. That lack of influence from
Downlev
provides the stability of the
Neutron while it is into the
NUC.
- Outside
the atom, the "free"
neutron,
that now is separated from the
atom's protons,
is no longer immune from the
influence of the environmental
Downlev.
As such, the
environmental
xenofluid
(eXF)
medium, by
Downlev,
will force the disintegration of the
neutron
by separating the naked electron
(nakel)
from the naked proton
(nakep).
Both now, the "naked"
electron
and the "naked"
proton
will gradually retake their
respective original states of a
"full-fledged" particles regaining
their charges:
|
with
the
electron
(regaining its
XB-cloud)
and
|
|
with
the
proton
becoming "active" again
(regaining its
coverlon
(COV)
mantle).
|
|
|
On
the "Temperature" of Chemical
Elements
|
 Electrons
(ELs)
surrounding the NUC
will "fall" into respective low-dense depressed
wave-like tracks and, as in a wave, they will
"rise" each time a proton of the
NUC
emits an ergon.
Because all protons within a stable
NUC
are outphased,
it follows that, at one given time, only one
ergon
will be emitted within the
NUC.
Electrons
(ELs)
surrounding the NUC
will "fall" into respective low-dense depressed
wave-like tracks and, as in a wave, they will
"rise" each time a proton of the
NUC
emits an ergon.
Because all protons within a stable
NUC
are outphased,
it follows that, at one given time, only one
ergon
will be emitted within the
NUC.
Depending
on the number of protons that a stable
NUC
has,
the emitted ergon
can be absorbed in full, partially or not all by
the NUC.
And that is because, in their influx
phases, the protons of a
NUC
can --depending of their number-- absorb
entirely, partially, or not at all, the released
ergon
of a saturated
proton that has reached its outburst
moment. In fact, the
NUC
containing only one single proton is the only
NUC
that cannot absorb or retain any portion of an
emitted ergon.
|
|
|
|
The
chemels
that are able to release in their
environment all of their produced
ergons,
in full, are the "hot"
chemels,
called hotons;
those that are able to release only a
bit (or a fragment) of their produced
ergons
are the "warm"
chemels,
called warmons;
and finally, those
chemels
that
are not able to release at all (totally
or partially) its produced
ergons
are the "cold"
chemels,
called
coldons.
|

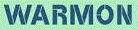
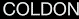
|
|
|
|
The
less number of protons a
NUC
has,
the "warmer" that chemel
is going to be. And that is because a
produced ergon-radiation
in the NUC
will be able to escape more readily
(totally or partially) into the
surrounding environment when it
encounters a weaker global
proton-influx
generated by a lesser number of
protons.
In the
reverse,
that is to recognize that the more
number of protons a
NUC
has, the "colder" that
chemel
is going to be due to the increase in
magnitude of the global
proton-influx
created that is able to absorb a
greater portion of the ergon-radiation.
As such,
the "hottest"
chemels
are the chemels
with the lowest number of protons:
|
with
the
Hydrogen
(1H)
atom being the sole
hoton
in Nature and,
followed with the
Helium
(2He)
atom by being the hottest
warmon.
|
|
|
|
|
|
|
|
BTW,
There is no accident that all
stars
are made of those two
"hottest" chemels:
H
and
He.
In addition, since those two
chemels
are the simplest elements of
Nature, they are also the most
abundant in the outer cosmic
space. The ergons
released from hotons
and warmons
generate also the
cosmic
background radiation
(COBAR).
|
|
With
the new blueprint of the atomic configuration
vested in RUBOT,
the next challenge at the horizon is to decipher
the main classification and characteristics of
chemels
--the subject contemplated for the next
page.
|
|
  
|

|
|